SPC-069/SPX-001 – Respiratory Infection treatment candidate
SpectrumX is progressing the first novel drug candidate in its pipeline. SPX-001 is currently in the clinical trial approval process for a Phase Ib clinical trial. SPX-001 is an inhaled treatment, with the drug candidate taken via a nebuliser which delivers the unique chemistry designed specifically for the respiratory system. This allows for a direct mode of action against viral particles both in the sinuses and lungs.

Initial trials will focus on the treatment on influenza which globally is responsible for 518,000 deaths per year.
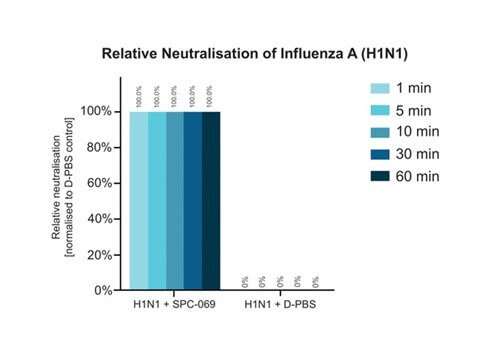
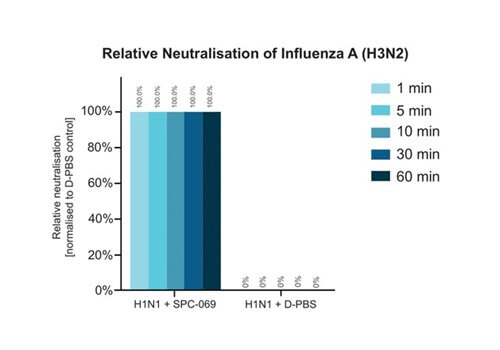
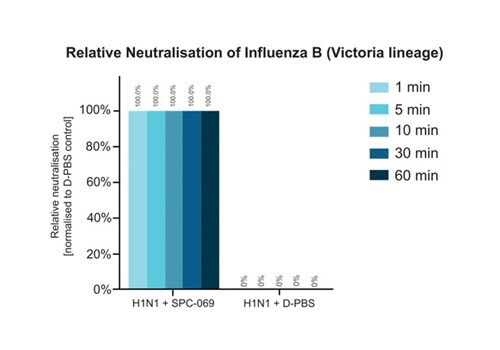
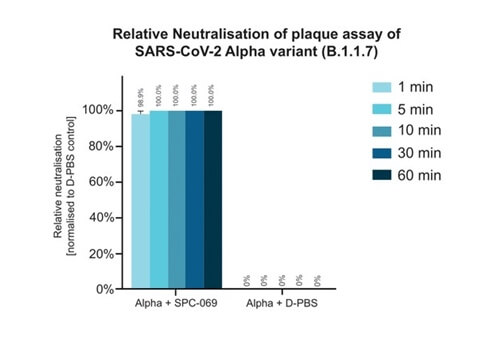
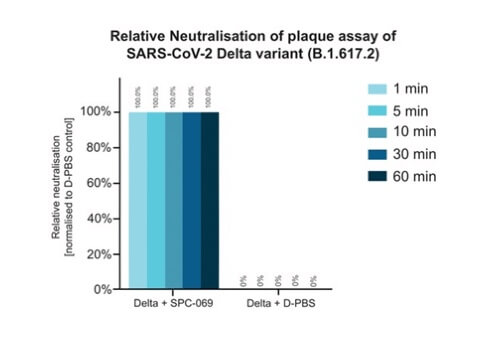
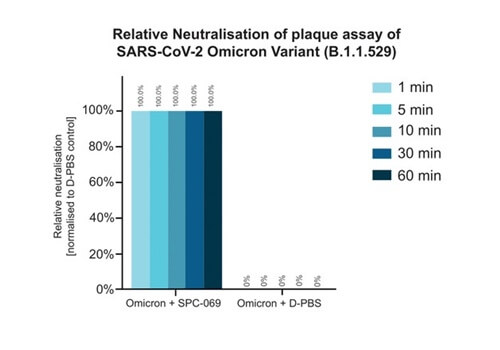
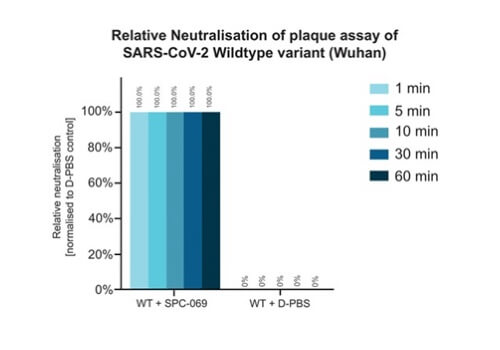
Due to the positively non-selective mode of action of the underlying active ingredient (HOCl), SpectrumX plans in time to undertake clinical trials for a range of widespread respiratory infections. In preparation for a Phase Ib Challenge Trial, SPC-069 (the active pharmaceutical ingredient in SPX-001) was tested in vitro against Influenza A (H1N1), Influenza A (H3N2) and Influenza B (Victoria Lineage), showing 100% eradication in 60 seconds.